UCB, a global biopharmaceutical company, has recently presented new interim data from BE BRIGHT, an open-label extension (OLE) trial of an interleukin (IL)-17A/F inhibitor ‘bimekizumab’ for plaque psoriasis (PsO), at the 2021 American Academy of Dermatology (AAD) Summer Meeting. While the data are still preliminary, they strongly support a potential approval for bimekizumab in PsO and will reinforce physicians’ positive opinions about the drug, according to GlobalData, a leading data and analytics company. Leia Mais »
International News
Anticoagulation strategy for COVID-19 patients and important insights gained from hospitalized cases: reveals GlobalData
The prevention of thrombosis is one of many priorities in managing the COVID-19 patients, but there are several complex considerations, especially when examining anticoagulation strategies. General considerations include the dose of anticoagulant, anticoagulant drug/drug class choice, and treatment duration, as well as to whom and when to administer anticoagulation along with the disease spectrum, according to GlobalData, a leading data and analytics company. Leia Mais »
Grünenthal’s Glucocorticoid Receptor Modulator enters clinical development
AACHEN, Germany, July 27, 2021 /PRNewswire/ — Grünenthal announced today that the first volunteers have been enrolled in a Phase I trial of its Glucocorticoid Receptor Modulator (GRM). The oral investigational medicine aims to provide a therapy option with broad anti-inflammatory efficacy and a more favourable benefit-risk profile compared to current glucocorticoid-based therapies like prednisolone.
The Phase I trial includes 80 healthy volunteers and is designed as a head to head comparison between the GRM and prednisolone, the most frequently used glucocorticoid.[1] Observing several biomarkers, the trial will assess the influence of the investigational medicine on bone metabolism and glucose levels. Reduced bone formation leading to osteoporosis as well as increased blood glucose levels leading to an increased risk of diabetes are among the most common side-effects of glucocorticoids, which are a strong limitation for their long-term use at highly effective doses.[2],[3],[4] The results of the study are expected to be available in the first quarter of 2022.
“Millions of patients rely on glucocorticoid-based therapies to manage their condition and are therefore at risk to experience severe side effects”, says Jan Adams, M.D., Chief Scientific Officer, Grünenthal. “We strive to increase the quality of life for these patients during long-term treatments by providing a therapy option that delivers a broad anti-inflammatory efficacy with significantly fewer side effects.”
The investigational medicine is a Grünenthal proprietary development and is currently the most advanced compound of the company’s Glucocorticoid Receptor Modulator programme. Preclinically, the compound has shown anti-inflammatory properties comparable to prednisolone with a lower risk of glucocorticoid receptor mediated side effects.
This Phase I trial is Grünenthal’s latest milestone in progressing its research pipeline. Recently, the company announced another Phase I trial with its Nociceptin/Orphanin Peptid Receptor (NOP)-agonist for the treatment of chronic neuropathic pain. In addition, Grünenthal announced several Phase III studies investigating the efficacy, safety and tolerability of Qutenza® (capsaicin) 8% topical system in post-surgical neuropathic pain (PSNP) and the efficacy, safety and tolerability of Resiniferatoxin in patients with pain associated with osteoarthritis of the knee. Both programmes are expected to start in 2021.
About Glucocorticoids
Glucocorticoids are a class of corticosteroids derived from the steroid hormone cortisol. Glucocorticoids are potent anti-inflammatory agents regardless of the cause of the inflammation and thus are used to suppress various allergic, inflammatory, and autoimmune disorders and to treat diseases caused by an overactive immune system. Long-term administration of Glucocorticoids can lead to a number of side effects, including osteoporosis and diabetes.
About Grünenthal
Grünenthal is a global leader in pain management and related diseases. As a science-based, privately-owned pharmaceutical company, we have a long track record of bringing innovative treatments and state-of-the-art technologies to patients worldwide. Our purpose is to change lives for the better – and innovation is our passion. We are focusing all of our activities and efforts on working towards our vision of a world free of pain.
Grünenthal is headquartered in Aachen, Germany, and has affiliates in 29 countries across Europe, Latin America and the US. Our products are available in more than 100 countries. In 2020, Grünenthal employed around 4,500 people and achieved sales of € 1.3 bn.
More information: www.grunenthal.com
Follow us on:
LinkedIn: Grunenthal Group
Instagram: grunenthal
[1] Robert A. Overman, Jun-Yen Yeh, and Chad L. Deal; Prevalence of Oral Glucocorticoid Usage in the United States: A General Population Perspective; Arthritis Care & Research, Vol. 65, No. 2; February 2013; pp 294 –29. DOI 10.1002/acr.21796
[2] Liu X, Zhu X, Miao Q, Ye H, Zhang Z, Li Y; Hyperglycemia Induced by Glucocorticoids in Nondiabetic Patients: A Meta-Analysis; Ann Nutr Metab 2014; 65: 324-332. doi: 10.1159/000365892
[3] Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O’Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T; American College of Rheumatology. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016 Jan;68(1):1-25. doi: 10.1002/acr.22783. Epub 2015 Nov 6. PMID: 26545825.
[4] Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, Tran TN; Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018; 11: 193-204 https://doi.org/10.2147/JAA.S176026
Healthcare companies see tech partnerships as necessary to implement smart technologies, according to a survey by GlobalData
As pharma companies continue to scale up digital transformation processes, a survey by GlobalData has highlighted that teaming up with experienced external vendors is driving innovative technology implementation. The leading data and analytics company notes that technologies such as 5G, IoT, sensors, blockchain, cloud and virtual/augmented reality were the top technologies for which pharma companies used external vendors, whereas they were more likely to develop artificial intelligence (AI), big data/analytics or robotics in house comparing to the rest of smart technologies. Leia Mais »
Byondis Appoints Timo van den Berg as Senior Director, Immuno-Oncology R&D
Byondis Appoints Timo van den Berg as Senior Director, Immuno-Oncology R&D
Nijmegen, the Netherlands – 15 July 2021– Byondis B.V. today announced the appointment of Timo K. van den Berg, Ph.D., as senior director, Immuno-Oncology (IO) Research and Development. Van den Berg will assume this role on September 1, operating out of Byondis’ headquarters in Nijmegen.
In his new role, Van den Berg will be instrumental in helping shape Byondis’ IO pipeline, which includes the anti-SIRPα monoclonal antibody BYON4228.
“We are pleased to welcome Timo to the Byondis family. He is a globally recognized IO pioneer, having made important contributions to the field, such as being the first to describe the role of SIRPα and recognizing the potential to use this target to develop a drug for the benefit of cancer patients,” said Byondis Chief Scientific Officer Wim Dokter, Ph.D.
As a longstanding Byondis collaborator, Professor van den Berg played a pivotal role in the discovery of the CD47-SIRPα axis as an innate immune checkpoint. CD47 is known in the IO space as the “don’t eat me” signal that allows tumors to escape recognition and destruction.
“I am very excited about this new chapter in my career,” said Professor van den Berg. “I believe that Byondis and I are well matched. We share the same values, including a strong, goal-oriented work ethic, and a passion for improving patients’ lives by creating precision medicines targeting difficult-to-treat cancers.”
Van den Berg brings to Byondis more than 30 years of experience in the IO field — as a renowned researcher, educator, inventor, lecturer and coauthor of over 175 peer-reviewed publications. His most recent position was head and principal investigator, Immunotherapy Laboratory, Department of Molecular Hematology at Sanquin Research, Amsterdam, the Netherlands. Since 2017, Van den Berg has been professor of Immunotherapy, Vrije Universiteit, which is affiliated with the Department of Molecular Cell Biology and Immunology, Amsterdam University Medical Center. He spent 25 years as research group leader and department head at Amsterdam University Medical Center and Sanquin Research. Sanquin, a non-profit organization responsible for the blood supply in the Netherlands, also develops and produces pharmaceutical products, conducts scientific research and develops and performs diagnostic services.
Van den Berg is a member of many boards, including Amsterdam Infection and Immunity Institute and Cancer Center Amsterdam. His professional memberships include the Dutch Society of Immunology and the European Society for Clinical Investigation. He received his Ph.D. from Amsterdam’s Vrije Universiteit, and his M.Sc. in Biological Sciences (cum laude) from the University of Amsterdam.
About Byondis Leia Mais »
Microbiome-targeting pipeline addresses multiple immunological indications, says GlobalData
Microbiome is a space with high potential, a fact underlined by the complete absence of microbiome-targeting treatments on the market. This therapeutic area is set to evolve significantly in the coming years as the current pipeline holds numerous potential candidates, with the majority still in early stages of drug development and only four in late-stage trials for gastrointestinal (GI), dermatological and respiratory indications, says GlobalData, a leading data and analytics company.
GlobalData’s report, ‘Thematic Research: Microbiome-Targeted Therapeutics in Immunology’, reveals that 23 microbiome-targeting therapeutics are currently being developed in the US and the *5EU for GI, dermatological, and respiratory conditions. Each of these therapies aims to address these diseases by manipulating the microbiome.
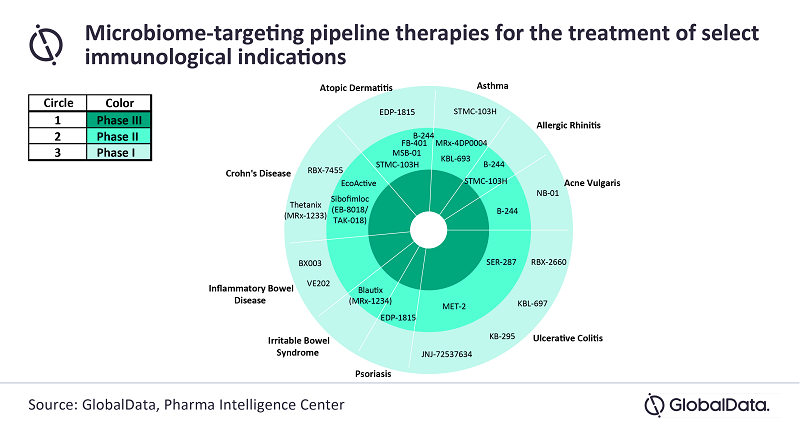
Source: Global Data.
Chris Pilis, Immunology Analyst at GlobalData, comments: “Most microbiome-targeting agents in the pipeline are in early stages of development: roughly half are in Phase I and half are in Phase II. Out of the 23 drugs, 13 are being investigated for GI indications, with the majority targeting ulcerative colitis (UC), whereas six and four are being developed for dermatological and respiratory conditions, respectively. Notably, many of the microbiome-targeting drugs for dermatological and respiratory indications are oral formulations, likely targeting these diseases via the gut-skin and gut-lung axes, respectively.”
The space is currently dominated by small- and mid-size biotech, however, Big Pharma players such as Takeda and Johnson & Johnson have shown increasing interest in this space lately with the development of the Phase II agent Sibofimloc (NCT03943446), and Phase I agent JNJ-72537634 (NCT03931447), respectively. Four therapies are in late-stage Phase II trials: 4D Pharma’s Blautix for irritable bowel syndrome (IBS), AOBiome’s B-244 for acne vulgaris and atopic dermatitis (AD), Evelo Biosciences’ EDP-1815 for psoriasis, and Seres Therapeutics’ SER-287 for UC. Of these agents, key opinion leaders interviewed by GlobalData were particularly enthusiastic about the prospects of 4D Pharma’s Blautix and AOBiome’s B-244, as both agents have already demonstrated promising data in Phase Ib or in early Phase II trials.
Pilis notes: “Decreased biodiversity and imbalance of bacterial levels in the gut microflora are involved in IBS manifestation, which is what Blautix is aiming to address. It is an oral formulation that releases single strains of commensal Blautia hydrogenotrophica that consume H2 gases causing bloating during the disease. The drug also induces acetate production, which promotes gut health, and increases microbiome diversity, ultimately trying to tackle both cause and effect of the disorder. Blautix is currently being evaluated in a Phase IIb trial (NCT03721107), but has exhibited encouraging efficacy and safety results in a previous Phase Ib trial.
“What is most promising about this candidate is that it is under investigation for both sub-groups of IBS-C and IBS-D patients, which until now have had very different therapeutic regimens. If successful, Blautix would be the first marketed product capable of treating both conditions. Consequently, this product could also be effective in treating IBS-M patients (one third of IBS cases) who to date have no treatments approved by the FDA or EMA. Ultimately, Blautix could have a strong impact on the market by being the first microbiome-targeting therapy for IBS and the first treatment approved for both IBS-C and IBS-D, which may potentially grant Blautix the exclusive market share of IBS-M patients.”
B-244 is a topical product under development by AOBiome for the treatment of acne and AD. GlobalData expects this product may gain popularity if marketed as a more ‘natural’ alternative to topical retinoids in acne and/or corticosteroids in AD. Thus far, B-244 has displayed good efficacy and tolerability for both acne and AD. It is currently in a Phase IIb trial (NCT04490109) for mild-to-moderate AD and has already been evaluated in a Phase IIb trial for mild-to-moderate acne (NCT02832063).
Pilis adds: “Although AOBiome read out positive data for NCT02832063 in 2017, the company has seemingly stalled development of B-244 in acne, possibly prioritizing launch of the drug in AD. If successful in clinical trials, B-244 will be the first skin microbiome-targeting drug to enter the market. GlobalData believes its unique mechanism of action and potential to manage two distinct conditions may lead to strong uptake by patients.
“Products such as Blautix and B-244 in particular are microbiome-targeting candidates setting high expectations, by either meeting the unmet needs of difficult-to-treat patients or by addressing multiple indications.”
*5EU: France, Germany, Italy, Spain, UK
Clarity Pharma unveils new website to support enhanced consultancy service offering
A dynamic and contemporary new website from Clarity Pharma, part of Clarity Global Group, demonstrates the breadth of services it offers to mid-market pharmaceutical and healthcare companies around the world seeking new opportunities in the UK healthcare market.
Olympic Games highlights global inequity in COVID-19 vaccination progress, says GlobalData
The 2020 Olympic Games in Tokyo will take place from July 23 to August 8, 2021, with a one-year delay due to the COVID-19 pandemic; however, it is unclear, yet, if the country and the world is ready for a global spectacle of that size, says GlobalData, a leading data and analytics company. Leia Mais »
Anti-amyloid therapies show potential for Alzheimer’s after breakthrough designation, says GlobalData
Eisai and Biogen’s lecanemab (BAN2401) and Eli Lilly’s donanemab have recently received breakthrough therapy designation (BTD) by the US Food and Drug Administration (FDA) for the treatment of Alzheimer’s disease (AD). The potential approval of lecanemab and donanemab will increase the number of disease-modifying treatments (DMTs) available that target and affect the underlying disease progression of AD, says GlobalData, a leading data and analytics company. Leia Mais »
AURORA study: the molecular changes driving metastatic breast cancer
First results of the European AURORA study: towards a better understanding of the molecular changes driving metastatic breast cancer
AURORA is an international academic research programme based on molecular screening and is dedicated to improving our understanding of metastatic breast cancer. Leia Mais »
 2A+ Farma Portal de notícias
2A+ Farma Portal de notícias
