Biogen’s tofersen has failed to achieve its primary endpoint in the Phase III VALOR study for amyotrophic lateral sclerosis (ALS). This setback is likely to hinder Biogen’s planned filing for United States Food and Drug Administration (FDA) approval in ALS. The company may take a similar approach as it did with its Alzheimer’s disease asset, Aduhelm (aducanumab), and still pursue an accelerated approval using some modestly encouraging data from the secondary endpoints of the trial, says GlobalData, a leading data and analytics company. Leia Mais »
International News
Addition of SGLT2 inhibitor with improved efficacy will be able to address demands of T2D market, says GlobalData
At the 57th annual European Association for the Study of Diabetes (EASD) 2021 meeting, results were presented for a 3-year prospective study. The study results demonstrated that SGLT2I, either empagliflozin (Jardiance) or dapagliflozin (Farxiga), are efficacious as part of a quadruple combination therapy with metformin, dipeptidyl peptidase-4 inhibitor (DPP-4I) and glimepiride in T2D patients. Additionally, SGLT2Is are able to address the demands of the T2D market for therapies that can be prescribed in combination to address the sub-optimal HbA1c control, says GlobalData, a leading data and analytics company.
The study examined the addition of sodium glucose cotransporter 2 inhibitor (SGLT2I) to the existing triple combination therapies in type 2 diabetes (T2D) patients that continue to have sub-optimal glycemic control.
Akash Patel, Pharma Analyst at GlobalData, comments: “Key opinion leaders (KOLs) interviewed by GlobalData have expressed significant interest in identifying further possible combination therapies that include SGLT2, especially for patients who are taking a combination of therapies but are unable to achieve optimal glycemic control or have developed additional cardiorenal comorbidities.”
The trial was a single-center, three-year, open-label, prospective observational study conducted in eligible patients ages 19 years and older who had sub-optimal glycemic control (hemoglobin A1c [HbA1c] 7.5–12.0%) despite the maximum doses of metformin, DPP-4I, and glimiperide, with stable dosage for at least three months. These patients were treated with empagliflozin at 25mg per day or dapagliflozin at 10mg per day as a fourth OAD to their existing triple combination therapy, at their physician’s discretion. A total of 262 patients were enrolled, split between empagliflozin (25mg per day, n=185) and dapagliflozin (10mg per day, n-177).
The efficacy outcomes included the change in HbA1c, fasting plasma glucose (FPG), and other cardiometabolic variables at the three-year point. The safety outcomes, hypoglycemia, volume depletion, nocturia, and genitourinary tract infections (GUTIs). The mean HbA1c reduction at the three-year point was -1.7% in the empagliflozin group versus -1.1% in the dapagliflozin group (P=0.001). There was a similar trend also observed with FPG (-59.3 ± 53.3mg/dL versus -47.4 ± 61.7mg/dL for empagliflozin and dapagliflozin, respectively, P=0.055).
The trial concluded that the efficacy of SGLTIs has been sustained for three years as part of a quadruple combination therapy. Blood glucose control was achieved more often with empagliflozin (25mg per day) than with dapagliflozin (10mg per day).
Mr. Patel concludes: “With SGLT2Is demonstrating significant efficacy in the treatment of cardiorenal comorbidities and having gained FDA and EMA approval for treatment in these areas, these inhibitors will be increasingly prescribed in both triple and quadruple combination therapies across the major markets. SGLT2Is are likely to continue to increase their T2D market share and become a leading therapeutic class.”
Source: Global Data.
Boehringer Ingelheim opens high-tech tablet production facility in Ingelheim
Ingelheim, Germany, October 27, 2021 – Rhineland-Palatinate Minister President Malu Dreyer was on hand as Boehringer Ingelheim opened a new tablet production facility in Ingelheim today. Starting immediately, the Solids Launch Factory will manufacture all the company’s new drugs introduced in tablet form (solids). The facility will employ around 75 workers. Total investments in the plant amount to EUR 90 million. Leia Mais »
Teva and MODAG Announce Licensing Collaboration for Neurodegenerative Disease Drug Candidate
( Tel Aviv, Israel & Wendelsheim, Germany, )
Anle138b targets pathological alpha-synuclein oligomers and is being evaluated in patients with neurodegenerative diseases for potential disease modification. Under the terms of the agreement and pending regulatory clearance, Teva will receive an exclusive global license to develop, manufacture and commercialize anle138b and sery433. The companies will jointly develop the compounds for the multiple system atrophy (MSA) and Parkinson’s disease (PD) indications based on early-stage clinical studies, and consider exploring additional indications based on clinical outcomes.
A Phase 1 (NCT04208152) study examining anle138b in healthy volunteers completed in July 2020 demonstrated favorable benefit-risk profile at all dose levels while achieving higher plasma levels than those required for full therapeutic efficacy in animal models. Anle138b was initially developed in patients with MSA and PD and has the potential to be applied to other neurodegenerative disorders, such as Alzheimer’s disease. A Phase 1b (NCT04685265) clinical trial evaluating the safety of the compound, as well as efficacy measures in patients living with PD, is currently being conducted.
“With Teva’s strong foundation in neuroscience and our in-house expertise in neurology and psychiatry, this licensing and collaboration agreement adds a promising new compound to our early-stage pipeline as a possible orphan disease treatment for the growing patient population living with multiple system atrophy, as well as a potential option for patients living with Parkinson’s disease,” said Hafrun Fridriksdottir, Executive Vice President, Global R&D. “We at Teva are excited about collaborating with the MODAG team and look forward to future developments as we continue to follow the science and explore additional indications for both partnered compounds.”
Dr. Matthias, CEO of MODAG added, “We are pleased to partner and work alongside Teva, an organization that has longstanding, extensive expertise in the development of therapeutics. In addition to the previous support we have received from the Michael J. Fox-Foundation and the Cure Parkinson’s Trust, this partnership further underscores the heightened potential of our lead candidates to do what no drug is currently capable of: blocking the progression of synucleinopathies. Building upon our notable preliminary results, we look forward to the continued development of anle138b alongside Teva to help patients living with currently untreatable neurodegenerative diseases, including MSA, PD and Alzheimer’s disease. With the introduction of small-molecule medication, we open a new chapter in the fight against neurodegenerative diseases and have the chance to improve the lives of millions of patients drastically.”
Multiple System Atrophy
Multiple system atrophy (MSA) is a rare neurodegenerative disorder classified clinically as “atypical parkinsonism” and belongs to the group of synucleinopathies. MSA is characterized histopathologically by abnormal deposits of the α-synuclein protein, mainly in oligodendroglial cells (glial cytoplasmic inclusions) and also in certain nerve cells. Typically, there is a dysfunction of the autonomic nervous system, i.e., disturbances of bladder function, erectile function, intestinal mobility, or the regulation of blood pressure in combination with a movement disorder. The movement disorder often presents with either Parkinson-like symptoms or a disturbance of cerebellar function, such as ataxia, gait and speech problems. In the US, EU, and Japan, MSA affects about 40,000 people (prevalence of 4 per 100,000), with about 6,000 new cases diagnosed each year (incidence of 0.6 per 100,000). Post-diagnosis life expectancy is about 7-10 years. Due to the relatively small number of affected patients and lack of effective therapy, MSA qualifies for orphan status, allowing a shorter development path. This unmet medical need for MSA disease modification is the driver to develop new therapies that could potentially impact the lives of patients.
New computer modelling could boost drug discovery
Scientists from Queen’s University Belfast have developed a computer-aided data tool that could improve treatment for a range of illnesses.
Boehringer Ingelheim Launches University of Medicine Excellence
Inaugural ’Accelerate’ program designed and delivered by Harvard Medical School Executive Education with the goal of accelerating clinical development and medical affairs Leia Mais »
AbbVie likely to retain key position in axial spondyloarthritis digital marketing space in Japan, says GlobalData
Historically, anti-tumor necrosis factor (TNF) drugs were the key biologic therapies available in Japan for axial spondyloarthritis (axSpA) treatment. However, the overall competition with the new class of marketed biologics such as anti-IL-17 therapies and the marketed biosimilars is expected to impact the sales of leading drugs — AbbVie’s Humira and Janssen’s/Mitsubishi Tanabe’s Remicade. Against this backdrop, leading and new players offer strong support to Japanese axSpA patients in the branded space through digital channels, says GlobalData, a leading data and analytics company. Leia Mais »
Arcutis may face challenges when marketing roflumilast despite commercial advantage, says GlobalData
Arcutis Biotherapeutics recently announced the submission of a New Drug Application (NDA) for its topical phosophodiesterase 4 (PDE4) inhibitor, roflumilast, in the treatment of mild-to-severe plaque psoriasis (PsO). Despite having a potential commercial advantage as the first branded topical therapy for an underserved PsO patient subgroup, GlobalData, a leading data and analytics company, notes that Arcutis may still face several challenges when marketing roflumilast. Leia Mais »
SGS Completes Expansion of Bioanalytical Testing Laboratory in France
SGS is proud to announce that the expansion of its bioanalytical laboratory in Poitiers, France is complete, with the addition of a new 800-m2 building to meet growing global demand for bioanalytical testing services to support drug development. Leia Mais »
COVID-19 shapes Indian pharma deals landscape, says GlobalData
India has a rapidly growing pharmaceutical market that, being the third largest pharmaceutical industry in the world by volume, holds a prominent place in global health. Over the last decade, the Indian pharmaceutical market has seen increasing domestic and foreign investment. While the overall number of deals fluctuated only slightly between 2011 and 2019, a considerable increase was confirmed for deals struck in 2020. Between 2019 and 2020, the deal number increased by 22%, likely triggered by the COVID-19 pandemic, reveals GlobalData, a leading data and analytics company.
Chris Pilis, Immunology Analyst at GlobalData comments: “Contract service agreements (CSAs), the most popular among the deal types in India, saw a strong increase in number—from 130 agreements in 2018 to 183 in 2019—and retained their elevated level through the next year. Spiking COVID-19 cases in 2020 drove large pharmaceutical companies to strike deals to outsource clinical research and manufacturing of anti-viral therapeutics and vaccines.
“Being the world’s largest vaccine producer, India was an optimal market to pursue these agreements. However, CSAs were not the only type of deal that saw an upward trend because of the pandemic; strategic alliances, including licensing agreements and partnerships, increased by 40% from 2019 to 2020. Most of these deals (30%) focused on COVID-19.”
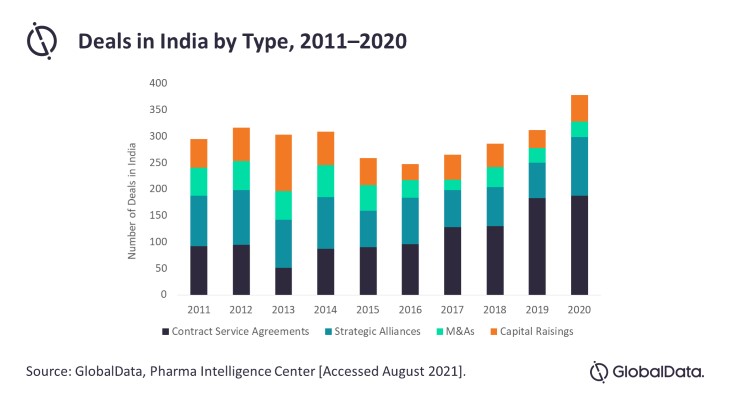
Other deal types were less popular. Mergers & acquisitions (M&As), even though common in the pharma industry, have remained low in number in India, averaging only 54 deals per year from 2011 and 2015 and dropping further between 2016 and 2020 to about 30 deals per year.
Mr Pilis continues: “The uncertainty brought about by the COVID-19 pandemic may have caused companies to continue to postpone more permanent relationships such as M&As, opting instead for more short-term or easily terminated CSAs and strategic alliances. Similarly, capital raising deals have remained low and static over the past five years. The minimal number of innovative drugs such as biologics (3% of the market) have probably prevented deals such as venture capital financing from taking place in India.”
Over the last decade, an increase in pharma deals has been observed in India because of its market expansion as well as the demand for novel therapeutics during the COVID-19 pandemic.
Mr Pilis concludes: “India’s leading role as the world’s top vaccine manufacturer has attracted many CSAs and strategic alliances in 2020. However, deals such as M&As and capital raises did not show similar COVID-19 pandemic-associated growth, possibly due to uncertainty and the limited innovation in fields such as biologics. The deals landscape highlights the current state of the India’s pharma market and will likely change substantially if the country shifts its focus more to innovation from its generic products industry.”
 2A+ Farma Portal de notícias
2A+ Farma Portal de notícias
