Immune checkpoint inhibitors (ICIs) have transformed the treatment of many late-stage cancers. This success has caused pharma companies to invest heavily into investigating their use in an earlier setting after a tumor has been removed by surgery (adjuvant), says GlobalData. The leading data and analytics company notes that, among these companies, Merck & Co has taken the most proactive steps for its mega blockbuster drug, Keytruda, to ensure dominance in the adjuvant setting.
Sakis Paliouras, PhD, Senior Oncology Analyst at GlobalData, comments: “So far, in most patients who receive any adjuvant treatment after surgery, that is going to be chemotherapy, radiation, or a combination of both. ICIs are widely anticipated to receive marketing authorizations in numerous adjuvant settings, and in many cancers, a first-mover advantage could bring billions in extra revenue.”
Currently, the highest-grossing ICI, Merck & Co’s Keytruda, which brought in $14.4bn in global revenue for 2020, has gained an FDA label in three adjuvant settings: melanoma, bladder cancer, and triple-negative breast cancer. Bristol Myers-Squibb’s (BMS) Opdivo also has three FDA labels in the adjuvant setting, while the nearest competitor, Roche’s Tecentriq, has gained only one label so far, in adjuvant treatment of non-small cell lung cancer (NSCLC), based on results from the IMpower010 trial.
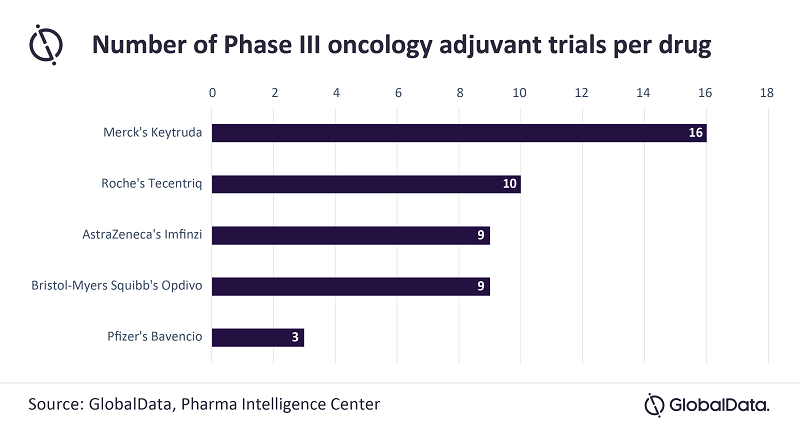
Paliouras continues: “An analysis of late-stage clinical trials in the adjuvant setting shows that Keytruda has the upper hand, with 16 ongoing clinical trials in various cancers, followed by Tecentriq with ten trials and Imfinzi/Opdivo with nine. However, AstraZeneca has taken a more proactive approach by sponsoring more trials than Roche, which has relied more on academic collaboration.
“Adjuvant treatment can be very lucrative and if any of these trials prove an extension in overall survival of patients, high physician adoption is to be expected. The area with the most adjuvant trials is cancers of the gastrointestinal tract, which is where the next big battle of ICIs is expected to be fought. Including liver, gastrointestinal, and colorectal cancer among others, this segment will include hundreds of thousands of patients worldwide eligible to be treated with ICIs.”
 2A+ Farma Portal de notícias
2A+ Farma Portal de notícias
