There are currently no Food and Drug Administration (FDA) approved treatments for eosinophilic esophagitis (EoE) in the US, says GlobalData. The leading data and analytics company notes that pipeline drugs with diverse mechanisms of action (MOAs) in late-stage development will create a dynamic market with a variety of treatment options for EoE patients in the near future.
In May 2020, The American Gastroenterological Association (AGA) partnered with the Joint Task Force (JTF) for Allergy-Immunology to publish new guidelines that provide recommendations for the management of pediatric and adult patients with EoE, including therapies that should be prioritized. According to these guidelines, the first-line approach for EoE management is dietary therapy, such as the elemental diet, allergy-testing elimination diet, and empiric elimination diet. The second-line option for EoE management is corticosteroid treatments such as fluticasone and budesonide. Lastly, dilation of strictures can help to provide short-term relief of dysphagia.
Mandana Emamzadeh, PhD, Healthcare Analyst at GlobalData, comments: “Currently, Takeda Pharmaceutical’s Eohilia (budesonide), an oral suspension, is in the pre-registration phase and will be the first marketed drug for EoE patients in the US. Budesonide received the European Medicines Agency (EMA) approval in November 2017. It is marketed under the brand name Jorveza as an orodispersible tablet for EoE.”
The flood of pipeline agents with different MOAs are expected to further expand the EoE treatment options in the US.
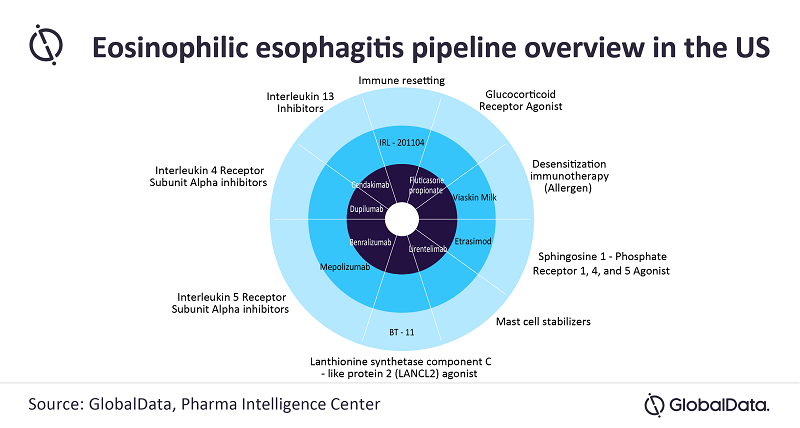
Sanofi and Regeneron are reportedly planning a regulatory filing for Dupixent (dupilumab), their blockbuster anti-inflammatory drug, after clearing the second Phase III trial.
Emamzadeh notes: “Dupixent, an interleukin 4 receptor subunit alpha inhibitor, has been granted a breakthrough therapy designation from the FDA due to a positive readout from the first Phase III trial in EoE. Additionally, the drug met its co-primary endpoints and showed significant improvements in clinical and histologic disease measures. As a result, GlobalData expects Dupixent to gain FDA approval in 2022.”
Other pipeline drugs with different MOAs in late-stage development include Ellodi Pharmaceuticals’ APT-1011 (fluticasone propionate), a glucocorticoid receptor agonist; Bristol-Myers Squibb and Celgene’s cendakimab, an interleukin 13 inhibitor; AstraZeneca’s Fasenra (benralizumab), an interleukin 5 receptor subunit alpha inhibitor; and Allakos’ lirentelimab, a mast cell stabilizer. Therefore, GlobalData anticipates a dynamic market with a variety of treatment options for EoE patients in the near future.
Source: GlobalData.
 2A+ Farma Portal de notícias
2A+ Farma Portal de notícias
